CBS2017 CALENDAR
Event Highlights
Pre-reading
Undeclared Veil of Secrecy Behind TCT2020
CBSMD

Byeong-Keuk Kim, MD, PhD, et al., used a prespecified cohort of 1,103 STEMI patients from 38 centers in South Korea who were enrolled in the broader TICO trial. Stratified randomization was performed based on the presence of STEMI, with 546 patients assigned to receive ticagrelor monotherapy after three-month DAPT and 557 patients assigned to receive ticagrelor-based 12-month DAPT. Results highlighted that major bleeding risk was reduced in the ticagrelor monotherapy group and there were no significant differences observed between the two treatment groups in terms of major adverse cardiac and cerebrovascular events. “This is the first report assessing the feasibility of the ticagrelor monotherapy after short-term DAPT for STEMI patients with [drug-eluting stent],” Kim said. However, he noted that “care should be taken in applying these results to the overall STEMI population, especially those at high risk for ischemia.”
CONTRIBUTION TO LITERATURE - The TICO trial showed that ticagrelor monotherapy after 3 months of DAPT was superior at preventing ischemia and bleeding after PCI for ACS.
Description - The goal of the trial was to evaluate ticagrelor monotherapy after 3 months of dual antiplatelet therapy (DAPT) compared with 12 months of DAPT after percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS).
STUDY DESIGN - randomized, parallel, stratification, open-label
Patients undergoing PCI for ACS were randomized to ticagrelor monotherapy after 3 months of DAPT (n = 1,527) versus standard therapy (n = 1,529).
- Total number of enrollees: 3,056
- Duration of follow-up: 12 months
- Mean patient age: 61 years
- Percentage female: 21%
- Percentage with diabetes: 27%
INCLUSION CRITERIA
- ACS treated with the ultrathin bioresorbable polymer sirolimus-eluting stent (Orsiro)
- ≥19 years of age
EXCLUSION CRITERIA
- >80 years of age
- Increased risk of bleeding
- Need for oral anticoagulation therapy
- Current or potential pregnancy
- Hepatic dysfunction
- Bradycardia
OTHER SALIENT FEATURES/CHARACTERISTICS
- Clinical presentation: unstable angina 29%, non-NSTEMI 35%, STEMI 36%
PRINCIPAL FINDINGS - The primary outcome, net adverse clinical events (death, MI, stent thrombosis, stroke, target vessel revascularization, or Thrombolysis in Myocardial Infarction [TIMI] major bleeding) at 12 months, occurred in 3.9% of the ticagrelor monotherapy after 3 months of DAPT group compared with 5.9% of the standard therapy group (p = 0.01). Association of ticagrelor monotherapy after 3 months of DAPT vs. standard therapy on the primary outcome: overall hazard ratio (HR) = 0.66, no multivessel disease HR = 0.41, multivessel disease HR = 0.86 (p for interaction = 0.04). Landmark analysis at 3 months for ticagrelor monotherapy vs. standard therapy for the primary outcome: (HR 0.41, p = 0.001).
SECONDARY OUTCOMES
- Major bleeding at 12 months: 1.7% of the ticagrelor monotherapy after 3 months group compared with 3.0% of the standard therapy group (p = 0.02)
- Stent thrombosis at 12 months: 0.4% of the ticagrelor monotherapy after 3 months group compared with 0.3% of the standard therapy group (p = 0.53)
- TICO-STEMI subgroup analysis: Major adverse cardiac and cerebrovascular events at 12 months: 2.7% of the ticagrelor monotherapy after 3 months group compared with 2.5% of the standard therapy group (p = 0.81)
INTERPRETATION - Among ACS patients who underwent PCI with an ultrathin biodegradable-polymer sirolimus-eluting stent, ticagrelor monotherapy after 3 months of DAPT was superior to standard therapy of DAPT for 12 months. Ticagrelor monotherapy was effective at preventing net composite ischemic and bleeding events. Bleeding events were defined by the TIMI criteria, which by way of reminder includes fatal bleeding, overt bleeding with drop in hemoglobin ≥5 g/dl or a 15% drop in hematocrit, and any intracranial hemorrhage.
This trial is like the similarly designed but placebo-controlled TWILIGHT trial. Neither of these trials addressed if aspirin monotherapy, instead of ticagrelor monotherapy in the 3- to 12-month period, would be equally effective. Ticagrelor monotherapy appears to be an emerging strategy, especially for patients with increased bleeding risk, after a short duration of DAPT.
RERERENCES
- Presented by Dr. Byeong-Keuk Kim at the Transcatheter Cardiovascular Therapeutics Virtual Meeting (TCT Connect), October 14, 2020.
- Presented by Dr. Byeong-Keuk Kim at the American College of Cardiology Virtual Annual Scientific Session Together With World Congress of Cardiology (ACC 2020/WCC), March 30, 2020.
PROSPECT II: NIRS-IVUS Imaging in AMI Patients
TCT 2020|LBCS Ⅰ
Intracoronary imaging of all three coronary arteries with a combination NIRS-IVUS catheter was safe in patients with acute myocardial infarction, based on findings from the PROSPECT II study presented Oct. 14 during TCT 2020.
David Erlinge, MD, PhD, FACC, et al., assessed data from 902 patients with recent STEMI (22.2%) and NSTEMI (77.8%) across centers in Denmark, Sweden and Norway. The median age was 63 and 17% were female. After successful treatment of all flow-limiting lesions with contemporary drug-eluting stents (DES), intravascular imaging was performed in the proximal 6-10 cm of all three coronary arteries, according to Erlinge. Untreated non-culprit lesions were prospectively identified by IVUS and their lipid content was assessed by blinded NIRS.
Results showed the primary outcome – major adverse cardiovascular events during median follow-up of 3.7 years – occurred in 14.4% of patients, of which 8% was caused by unanticipated events arising from untreated non-flow-limiting plagues and 4.6% caused by recurrent events at treated culprit lesions. The primary safety outcome – intravascular imaging-related major complications requiring treatment – occurred in two patients (0.2%). Erlinge, et al., note that NIRS identified “lipid-rich angiographically mild non-flow-limiting plaques that were responsible for future coronary events.”
PROSPECT ABSORB
PCI of angiographically mild lesions with large plaque burden was safe, substantially enlarged the follow-up minimum lumen area (MLA), and was associated with favorable long-term clinical outcomes, said researchers presenting findings from the PROSPECT ABSORB Trial Oct. 14 during TCT 2020.
The study, which was simultaneously published in the Journal of the American College of Cardiology, used three-vessel imaging and a combination intravascular ultrasound (IVUS) and near-infrared spectroscopy (NIRS) catheter in 898 patients presenting with myocardial infarction following successful PCI of all flow-limiting coronary lesions. Those patients with an angiographically non-obstructive stenosis not intended for PCI but with IVUS plaque burden ≥65% (N=192) were randomized to treatment of the lesion with either a bioresorbable vascular scaffold (BVS) plus guideline-directed medical therapy (GDMT) or GDMT alone.
The primary powered effectiveness endpoint was the IVUS-derived MLA at protocol-driven 25-month follow-up. Additionally, the primary safety endpoint was randomized target lesion failure at 24 months and the secondary clinical effectiveness endpoint was randomized lesion-related major adverse cardiac events (MACE) at latest follow-up.
Results showed the median angiographic diameter stenosis of the randomized lesions was 41.6%, while the NIRS-IVUS median plaque burden was 73.7%. The median MLA was 2.9 mm2, and median maximum lipid plaque content was 33.4%. Angiographic follow-up at 25 months was completed in 167 patients. The follow-up MLA in BVS-treated lesions was 6.9±2.6 mm2 compared with 3.0±1.0 mm2 in those treated with GDMT alone. Researchers also noted that the rates of target lesion failure at 24 months were similar across both groups. Randomized lesion-related MACE occurred in 4.3% of patients in the BVS-treated group compared with 10.7% in the GDMT-alone group (P=0.12).
Among the limitations to the study: it was not powered for clinical outcomes, and the present PCI results only apply to the first generation everolimus-eluting Absorb BVS. “Whether the results would be superior with a thinner-strut BVS or a contemporary metallic DES is unknown,” said Gregg W. Stone, MD, FACC. However, Stone and colleagues note that the “the favorable randomized lesion-related MACE rates observed after BVS treatment compared with GDMT alone warrants the performance of an adequately powered randomized trial to determine whether PCI treatment of focal vulnerable plaques improves patient outcomes.” But they also add, that “until such a study, PCI of non-ischemic lesions, even those with high-risk morphologic features, cannot be routinely recommended.”
The PROSPECT ABSORB study was embedded in the PROSPECT II study, also presented at TCT 2020.
CONTRIBUTION TO LITERATURE - PCI of proximal non–flow-limiting stenosis with angiographic stenosis <70%, FFR/iFR negative, and plaque burden on IVUS ≥65% with Absorb BVS resulted in a larger MLA on IVUS follow-up, with no difference in clinical endpoints at 24 months.
DESCRIPTION - The goal of the trial was to assess the safety and efficacy of percutaneous coronary intervention (PCI) of non–flow-limiting stenosis with Absorb bioresorbable vascular scaffold (BVS) compared with control, using near-infrared spectroscopy–intravascular ultrasound (NIRS-IVUS) to understand plaque burden.
STUDY DESIGN - Eligible
patients were randomized in a 1:1 fashion to either PCI with Absorb BVS
(n = 93) or control (n = 89). Both arms got optimal guideline-directed
medical therapy (GDMT).
Patients
undergoing PCI for ACS were randomized to ticagrelor monotherapy after 3
months of DAPT (n = 1,527) versus standard therapy (n = 1,529).
- Total number of enrollees: 182
- Duration of follow-up: 4.1 years
- Mean patient age: 64years
- Percentage female: 18%
- Percentage with diabetes: 11%
INCLUSION CRITERIA
- Successful PCI of all flow-limiting lesions
- Angiographic diameter stenosis <70% (with negative fractional flow reserve [FFR] or instantaneous wave-free ratio [iFR] required if diameter stenosis was >40%)
- Reference vessel diameter 2.5-4.0 mm
- Lesion length ≤50 mm
- IVUS plaque burden ≥65%
PRINCIPAL FINDINGS
- The primary endpoint, MLA on IVUS, for PCI + GDMT vs. GDMT alone, was 6.9 vs. 3.0 mm2 (p < 0.0001).
- Across the entire lesion (including 5 mm margins): 5.2 vs. 2.9 mm2 (p < 0.0001)
SECONDARY ENDPOINTS for IVUS, for PCI + GDMT vs. GDMT alone
- Target lesion failure: 4.3% vs. 4.5% (p = 0.96)
- Target vessel myocardial infarction (TV-MI): 3.3% vs. 1.1%
- Lesion-related major adverse cardiac events (MACE): 4.3% vs. 10.7% (p = 0.12)
- Scaffold thrombosis: 1.1% vs. 0%
INTERPRETATION - The results of this pilot trial indicate that PCI of proximal non–flow-limiting stenosis with angiographic stenosis <70%, FFR/iFR negative, and plaque burden on IVUS ≥65% with Absorb BVS resulted in a larger MLA on IVUS follow-up, with no difference in clinical endpoints at 24 months. MACE rates were numerically lower with Absorb BVS PCI, while TV-MI rates were slightly higher.
RERERENCES
- Presented by Dr. Gregg Stone at the Transcatheter Cardiovascular Therapeutics Virtual Meeting (TCT Connect), October 14, 2020.
COMBINE (OCT - FFR)
TCT 2020|LBCS Ⅰ
Combined fractional flow reserve (FFR) and optical coherence tomography (OCT) can improve the accuracy of predicting adverse event outcomes in patients with diabetes mellitus (DM), according to Elvin Kedhi, MD, presenting results from the COMBINE (OCT-FFR) trial Oct. 14 during TCT 2020.
As part of the trial, 500 DM patients with stable or acute coronary syndromes and who had ≥1 (non-culprit) target lesion with a 40-80% diameter stenosis underwent FFR assessment. FFR-negative patients underwent OCT assessment and were given further medical treatment. Patients were then divided into two groups depending on the presence (Group A) or absence of (Group B) a thin cap fibroatheroma (TCFA). Patients with FFR-positive target lesions were revascularized and placed in a third group (Group C). The primary endpoint was a composite of cardiac death, target-lesion myocardial infarction, clinically-driven target lesion revascularization or hospitalization due to unstable angina at 1.5 years between groups A and B.
COMBINE (OCT-FFR) showed for the first time that in patients with DM more than 25% of all FFR-negative lesions represent high-risk plaques, said Kedhi, who also noted that the presence of TCFA appears to be a strong predictor of future major adverse cardiac events, despite lack of ischemia. Additionally, Kedhi and colleagues observed that patients with TCFA had a significant increase in target-lesion-related major adverse cardiovascular events and myocardial infarction compared with patients without TCFA.
Kedhi, et al., note that their findings offer new insights into the treatment of DM patients who tend to have worse outcomes in terms of FFR-negative lesions compared with those without DM. They also suggest that “ischemia and future adverse events represent, to a large extent, two separate concepts,” and should be studied further.
COMPARE CRUSH
TCT 2020|LBCS Ⅰ
CONTRIBUTION TO LITERATURE - In the COMPARE CRUSH trial, crushed prasugrel did not improve TIMI 3 flow at first angiography or complete ST-segment resolution at 1 hour post-PCI compared with integral prasugrel, both of which were administered as a 60 mg load in the ambulance prior to PPCI among patients with suspected STEMI.
DESCRIPTION - The goal of the trial was to assess the efficacy of prehospital crushed vs. integral prasugrel among ST-segment elevation myocardial infarction (STEMI) patients being considered for primary percutaneous coronary intervention (PPCI).
STUDY DESIGN - Eligible patients were randomized in a 1:1 fashion to either crushed (n = 369) or integral (n = 358) tablets of 60 mg prasugrel in the ambulance. They also received aspirin and heparin. Rapamycin target eluting stent (Firehawk MicroPort Medical, Shanghai, China) was used as the preferred stent.
- Total screened: 1,669
- Total number of enrollees: 727
- Duration of follow-up: 48 hours
- Mean patient age: 62 years
- Percentage female: 23%
INCLUSION CRITERIA
- Suspected STEMI and symptom onset within 6 hours
- Initially managed by a mobile emergency medical care unit
- Plan for PPCI
EXCLUSION CRITERIA
- History of a cerebral vascular accident
- Recent gastrointestinal bleeding
- Recent major surgery
- Indication for chronic oral anticoagulation therapy
- Dependent on hemodialysis
- Unable to swallow oral medication
- Presented with cardiogenic shock or cardiac arrest
OTHER SALIENT FEATURES/CHARACTERISTICS
- Symptom onset to first medical contact: 58 minute
- Anterior MI: 38%
- Use of glycoprotein inhibitors: 12%, opioids: 15%
PRINCIPAL FINDINGS
-The primary endpoint, Thrombolysis in Myocardial Infarction (TIMI) 3 flow in the infarct-related artery at first angiography, between crushed vs. integral prasugrel, was 31.0% vs. 32.7% (p = 0.64).
- Complete ST-segment resolution 1-hour post-PPCI: 59.9% vs. 57.3% (p = 0.55)
- Across the entire lesion (including 5 mm margins): 5.2 vs. 2.9 mm2 (p < 0.0001)
SECONDARY ENDPOINTS for crushed vs. integral prasugrel
- High platelet reactivity at start of PCI (P2Y12 inhibitor reactivity units >208): 43.3% vs. 62.6% (p < 0.01)
- Any bleeding: 3.3% vs. 3.9% (p = 0.63)
- Stent thrombosis: 0.6% vs. 0.7% (p = 1.0)
INTERPRETATION - The results of this trial indicate that crushed prasugrel did not improve TIMI 3 flow at first angiography or complete ST-segment resolution at 1 hour post-PCI compared with integral prasugrel, both of which were administered as a 60 mg load in the ambulance prior to PPCI among patients with suspected STEMI. Platelet reactivity was lower in the crushed prasugrel arm, but this did not translate into lower stent thrombosis events or need for less frequent bailout glycoprotein inhibitor. The trial was underpowered for the latter two endpoints.
There was no use of cangrelor in this study, which showed a reduction in intraprocedural and early stent thrombosis events compared with clopidogrel in the CHAMPION PHOENIX trial. In the ATLANTIC trial, no difference in myocardial perfusion markers was noted with prehospital vs. in-hospital administration of ticagrelor, although there was a benefit noted in stent thrombosis.
RERERENCES
- Presented by Dr. Georgios Vlachojannis at the Transcatheter Cardiovascular Therapeutics Virtual Meeting (TCT Connect), October 14, 2020.
ULTIMATE - 3 Year Follow-Up
TCT 2020|LBCS II
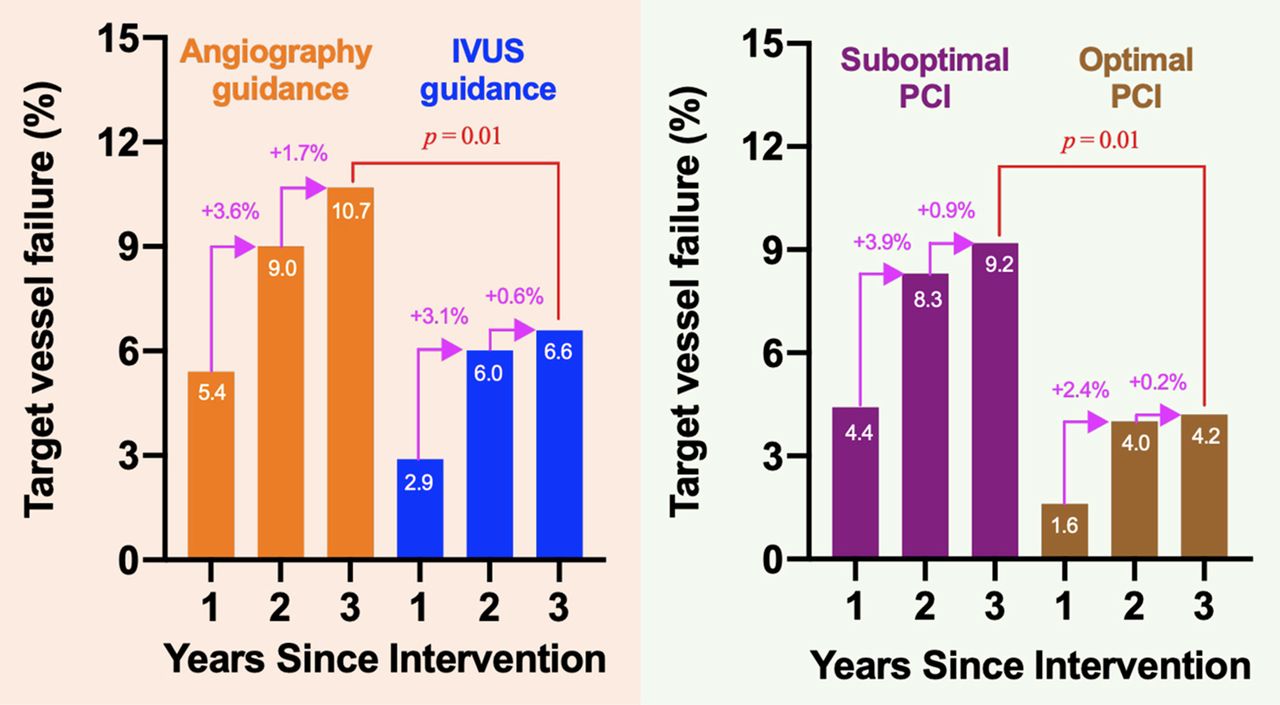
CONTRIBUTION TO LITERATURE -
The ULTIMATE trial showed that IVUS-guided PCI was superior to angiography-guided PCI at preventing target vessel failure.
DESCRIPTION - The goal of the trial was to evaluate intravascular ultrasound (IVUS)-guided percutaneous coronary intervention (PCI) compared with angiography-guided PCI among an all-comers group of patients undergoing PCI.
STUDY DESIGN - Randomized, Parallel
Patients undergoing coronary revascularization were randomized to IVUS-guided PCI (n = 724) versus angiography-guided PCI (n = 724).
“Optimal IVUS-guided PCI” characteristics: minimal cross-sectional area >5.0 mm2 (or 90% of distal reference lumen cross-sectional area), plaque burden at proximal and distal stent edges <50%, no edge dissection involving media with length >3 mm.
- Total number of enrollees: 1,448
- Duration of follow-up: 12 months
- Mean patient age: 65 years
- Percentage female: 26%
- Percentage with diabetes: 31%
INCLUSION CRITERIA
- All-comers group of patients undergoing PCI
- Had silent ischemia, stable or unstable angina, or myocardial infarction (MI; including both ST-elevation and non-ST-elevation MI) >24 hours from the onset of chest pain to admission
- Had de novo coronary lesion eligible for DES implantation
EXCLUSION CRITERIA
- Co-morbidity with life expectancy <12 months
- Intolerant of antithrombotic therapy
- Significant anemia, thrombocytopenia, or leucopenia
- History of major hemorrhage (intracranial, gastrointestinal, etc.)
- Chronic total occlusion lesion in either left anterior descending, left circumflex, or right coronary artery not re-canalized
- Severe calcification needing rotational atherectomy
PRINCIPAL FINDINGS - The primary outcome, target vessel failure at 12 months (cardiac death, MI, or target vessel revascularization), occurred in 2.9% of the IVUS-guided PCI group compared with 5.4% of the angiography-guided PCI group (p = 0.019). Among those who met the criteria for optimal IVUS-guided PCI, there appeared to be enhanced benefit from the use of IVUS compared with angiography-guided PCI.
SECONDARY OUTCOMES
- Cardiac death: 0.7% of the IVUS-guided PCI group vs. 1.4% of the angiography-guided PCI group (p = 0.19)
- MI: 1.0% of the IVUS-guided PCI group vs. 1.5% of the angiography-guided PCI group (p = 0.34)
- Target vessel revascularization: 1.5% of the IVUS-guided PCI group vs. 2.9% of the angiography-guided PCI group (p = 0.07)
- Definite/probable stent thrombosis: 0.1% of the IVUS-guided PCI group vs. 0.7% of the angiography-guided PCI group (p = 0.10)
LONG-TERM OUTCOMES - Target vessel failure (cardiac death, target vessel MI, or clinically driven target vessel revascularization) at 3 years: 6.6% of the IVUS-guided PCI group vs. 10.7% of the angiography-guided PCI group (p = 0.01)
INTERPRETATION - Among an all-comers group of patients undergoing PCI, IVUS-guided PCI was beneficial. IVUS-guided PCI was associated with a lower frequency of target vessel failure up to 3 years compared with angiography-guided PCI. All components of the composite outcome were numerically lower in the IVUS-guided PCI group; however, the greatest benefit seemed to be at reducing target vessel revascularization. Multiple randomized trials now support the use of IVUS guidance in optimizing coronary stent implantation as a mechanism to reduce adverse cardiac events.
REFERENCES -
- Presented by Dr. Junjie Zhang at the Transcatheter Cardiovascular Therapeutics Virtual Meeting (TCT Connect), October 15, 2020.
- Zhang J, Gao X, Kan J, et al. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: The ULTIMATE Trial. J Am Coll Cardiol 2018;72:3126-37.
- Editorial Comment: di Mario C, Koskinas KC, Räber L. Clinical Benefit of IVUS Guidance for Coronary Stenting: The ULTIMATE Step Toward Definitive Evidence? J Am Coll Cardiol 2018;72:3138-41.
- Presented by Dr. Junjie Zhang at the Transcatheter Cardiovascular Therapeutics meeting (TCT 2018), San Diego, CA, September 24, 2018.
XIENCE Short DAPT - 3 Year Follow-Up
TCT 2020|LBCS II
Among patients at a high risk of bleeding undergoing PCI with the XIENCE stent, a short dual antiplatelet therapy (DAPT) regimen of one or three months was noninferior to standard DAPT up to 12 months in terms of ischemic outcomes, according to findings from the XIENCE Short DAPT Program presented Oct. 15 during TCT 2020.
Presenting investigators Roxana Mehran, MD, FACC, and Marco Valgimigli, MD, PhD, also noted similar rates of clinically relevant (BARC 2-5) bleeding, with a significant reduction in major bleeding (BARC 3-5), and very low incidence of stent thrombosis.
Overall, the study aimed to evaluate the safety (all death or myocardial infarction) of a short DAPT regimen compared with a longer one, as well as determine the impact of short DAPT on clinically relevant bleeding and evaluate stent thrombosis. It was not a randomized trial. Key inclusion criteria included: history of stroke, chronic kidney disease, anemia, major bleeding in the last 12 months, and over the age of 75.
Mehran and Valgimigli noted several limitations to the study, including that the findings may not be generalizable to patients who do not meet the study’s inclusion/exclusions criteria. Additionally, “the observed treatment effect only applies to patients ‘free’ from adverse events and adherent to the DAPT treatment regimen for the first one to three months,” they added.
SCOPE II
TCT 2020|LBCS II
CONTRIBUTION TO LITERATURE - In the SCOPE 2 trial, TAVR with the Acurate neo valve did not meet criteria for noninferiority compared with CoreValve Evolut valve for treatment of symptomatic severe aortic stenosis in mostly intermediate-risk patients.
DESCRIPTION - The goal of the trial was to compare the safety and efficacy of TAVR with two self-expanding supra-annular valves, Acurate neo vs. CoreValve Evolut, among patients with severe aortic stenosis undergoing transfemoral TAVR.
STUDY DESIGN - Eligible patients were randomized in a 1:1 fashion to transfemoral TAVR with either Boston Scientific’s Acurate neo (n = 398) or Medtronic’s CoreValve Evolut (n = 398).
- Total number of enrollees: 1,448
- Duration of follow-up: 1 year
- Mean patient age: 83 years
- Percentage female:68%
INCLUSION CRITERIA
- Age ≥75 years
- Severe symptomatic aortic stenosis
- High risk for surgical AVR (SAVR) based on risk scores and/or heart team recommendation
- Aortic annulus dimensions and peripheral access suitable for either device
EXCLUSION CRITERIA
- Congenital or noncalcific anomaly of aortic valve, or unicuspid or bicuspid valve
- Severe left ventricular dysfunction
- Left-sided prosthetic valve
- Active infection
- Severe eccentric calcification
- Severe coagulation disorder
OTHER SALIENT FEATURES/CHARACTERISTICS
- Society of Thoracic Surgeons-Predicted Risk of Mortality (STS-PROM) score: 4.6%
- Annulus perimeter: 73.5 mm, aortic annulus area: 425 mm2
- General anesthesia: 13%
- Predilation: 79% (Acurate neo) vs. 41% (CoreValve Evolut)
PRINCIPAL FINDINGS - The primary endpoint, death or stroke at 1 year, for Acurate neo vs. CoreValve Evolut, was 15.8% vs. 13.9% (p = 0.055 for noninferiority).
- All-cause mortality: 13% vs. 9% (p = 0.13)
- Stroke: 5% vs. 6% (p = 0.33)
SECONDARY OUTCOMES
- New pacemaker implantation: 11% vs. 18% (p = 0.004)
- Cardiac death: 8% vs. 4% (p = 0.01)
- Access site complication: 9% vs. 6% (p = 0.22)
- ≥ Mild paravalvular leak at 30 days: 72.8% vs. 55.1% (p < 0.0001)
INTERPRETATION - The results of this trial indicate that TAVR with the Acurate neo valve did not meet criteria for noninferiority compared with CoreValve Evolut valve for treatment of symptomatic severe aortic stenosis in mostly intermediate-risk patients. Both are self-expanding, supra-annular valves with porcine pericardial leaflets. Cardiac death and paravalvular leak were higher with Acurate neo, while pacemaker rates were lower.
These data represent one of the few head-to-head comparisons between TAVR valves, and are important for the field. In the SCOPE-1 trial, Acurate neo was inferior to Sapien S3 for the primary endpoint. Although widely used outside the United States, the Acurate neo valve is not currently Food and Drug Administration (FDA) approved in the United States.
REFERENCES
- Capodanno D, Tamburino C, Bleiziffer S, et al. Comparison of self-expanding bioprostheses for transcatheter aortic valve replacement in patients with symptomatic severe aortic stenosis: the SCOPE 2 randomized clinical trial. Circulation 2020;Oct 15:[Epub ahead of print].
- Presented by Dr. Corrado Tamburino at the Transcatheter Cardiovascular Therapeutics Virtual Meeting (TCT Connect), October 15, 2020.
MITHRAS
TCT 2020|LBCS II
CONTRIBUTION TO LITERATURE - In the MITHRAS trial, routine closure of iatrogenic ASDs post-TMVr does not result in improved 6-minute walk distance or an improvement in mortality/HF hospitalization compared with conservative management, despite a normalization in Qp:Qs (baseline Qp:Qs ≥1.3; mean 1.5).
DESCRIPTION - The goal of the trial was to assess the efficacy of routine closure of persistent iatrogenic atrial septal defect (ASD) with relevant left-to-right shunting and a Qp:Qs of ≥1.3 among patients who had undergone trans-septal transcatheter mitral valve repair (TMVr).
STUDY DESIGN - Patients with persistent iatrogenic ASD and relevant left-to-right shunting (Qp:QS ≥1.3) 1 month post-TMVr were randomized in an open-label 1:1 fashion to either percutaneous ASD closure (n = 40) or control (n = 40). Closure was performed with Figulla Flex II, Occlutech, Jena, Germany.
- Left-to-right shunting with Qp:QS ≥1.3
INCLUSION CRITERIA
- Age ≥75 years
EXCLUSION CRITERIA
- Presence of interatrial shunt prior to TMVr
- No TMVr success
- Additional valvular heart disease planned for surgery or intervention
- Malignancy with expected survival <12 months
- Anatomic considerations precluding transcatheter ASD closure
OTHER SALIENT FEATURES/CHARACTERISTICS
- EuroSCORE II: 5.2%
- Residual mitral regurgitation grade 0/1: 78%, 2+: 20%
- Residual tricuspid regurgitation grade: 0/1: 52%, 2: 34%
- Left ventricular ejection fraction: 38%
PRINCIPAL FINDINGS - The primary endpoint, change in 6-minute walk distance at 5 months, for ASD closure vs. conservative management, was 5 vs. -1 m (p = 0.76).- All-cause mortality: 13% vs. 9% (p = 0.13)
SECONDARY ENDPOINTS for ASD closure vs. conservative management -
- Change in N-terminal pro-B-type natriuretic peptide from baseline: -846 vs. -279 pg/ml (p = 0.44)
- Change in Qp:Qs from baseline: -0.5 vs. -0.2 (p = 0.02)
- Mortality or heart failure (HF) hospitalization at 1 year: 21% vs. 23% (p = 0.22)
INTERPRETATION - The results of this trial indicate that routine closure of iatrogenic ASDs post-TMVr does not result in improved 6-minute walk distance or an improvement in mortality/HF hospitalization compared with conservative management, despite a normalization in Qp:Qs (baseline Qp:Qs ≥1.3).
This is a helpful trial despite its small sample size since it addresses a commonly encountered clinical scenario. ASD closure may still be warranted for large defects/tears caused by trans-septal access during TMVr. It is also possible that a persistent shunt may have some beneficial effects in patients with significant residual mitral regurgitation by allowing for left atrial decompression into the right side.
REFERENCES
- Presented by Dr. Philipp Lurz at the Transcatheter Cardiovascular Therapeutics Virtual Meeting (TCT Connect), October 15, 2020.
THE DEFINE PCI
TCT 2020|LBCS II
OBJECTIVES - This study sought to evaluate the incidence and causes of an abnormal instantaneous wave-free ratio (iFR) after angiographically successful percutaneous coronary intervention (PCI).
BACKGROUND - Impaired coronary physiology as assessed by fractional flow reserve is present in some patients after PCI and is prognostically relevant.
METHODS - DEFINE PCI (Physiologic Assessment of Coronary Stenosis Following PCI) was a multicenter, prospective, observational study in which a blinded iFR pull back was performed after angiographically successful PCI in 562 vessels in 500 patients. Inclusion criteria were angina with either multivessel or multilesion coronary artery disease with an abnormal baseline iFR. The primary endpoint of the study was the rate of residual ischemia after operator-assessed angiographically successful PCI, defined as an iFR <0.90. The causes of impaired iFR were categorized as stent related, untreated proximal or distal focal stenosis, or diffuse atherosclerosis.
RESULTS - An average of 1.1 vessels per patient had abnormal baseline iFRs, with a mean value of 0.69 ± 0.22, which improved to 0.93 ± 0.07 post-PCI. Residual ischemia after angiographically successful PCI was present in 112 patients (24.0%), with a mean iFR in that population of 0.84 ± 0.06 (range 0.60 to 0.89). Among patients with impaired post-PCI iFRs, 81.6% had untreated focal stenoses that were angiographically inapparent, and 18.4% had diffuse disease. Among the focal lesions, 38.4% were located within the stent segment, while 31.5% were proximal and 30.1% were distal to the stent. Post-PCI vessel angiographic diameter stenosis was not a predictor of impaired post-procedural iFR.
CONCLUSIONS - Blinded post-PCI physiological assessment detected residual ischemia in nearly 1 in 4 patients after coronary stenting despite an operator-determined angiographically successful result. Most cases of residual ischemia were due to inapparent focal lesions potentially amenable to treatment with additional PCI. (Physiologic Assessment of Coronary Stenosis Following PCI [DEFINE PCI]; NCT03084367)

REFERENCE
FORECAST
TCT 2020|LBCS III
In the FORECAST randomized clinical trial, the use of fractional flow reserve management derived from computed tomography (FFRCT) did not significantly reduce costs but did reduce the use of invasive coronary angiography (ICA).
FFRCT is a novel, validated, non-invasive method for describing both the amount of coronary atheroma from a CT coronary angiogram (CTCA), but also vessel-specific ischemia derived from the CTCA and other clinical parameters using a fluid dynamics computer model. Previous studies have indicated that FFRCT reduces the uptake of invasive angiography that shows no significant CAD, without compromising patient safety. The clinical effectiveness and economic impact of using FFRCT instead of other tests in the evaluation of patients with stable chest pain has not yet been tested in a randomized trial, although based upon cost models on observational data, FFRCT is already recommended in routine clinical practice by National Institute for Health and Care Excellence (NICE) in the UK, because it appeared cost dominant.
The primary endpoint of the FORECAST trial was resource utilization derived from non-invasive cardiac tests, invasive angiography, coronary revascularization, hospitalization for a cardiac event, and cardiac medications at nine months. Prespecified secondary endpoints included major adverse cardiac and cerebrovascular events, revascularization, angina severity, and quality of life (QOL).
In the trial, 1,400 patients with stable chest pain at 11 UK centers were randomized to receive either CCTA with FFRCT of lesions with stenosis severity of 40% or greater (test arm, n=699) or routine assessment as directed by the NICE Guideline for Chest Pain of Recent Onset (reference arm, n=700). The routine assessment arm included a mixture of non-invasive tests, including CCTA (without FFRCT) in 61.4% of subjects. The mean age of the overall population was 60 (25-89) years and 52% were male. Baseline demographics, angina status, and QOL/health status were similar between the groups.
In patients presenting with new onset stable chest pain, a strategy of CTCA with FFRCT, when compared with a strategy of routine care, did not significantly reduce average total costs in the NHS system (£1,605.50 vs. £1,491.46, p=0.962). At nine months, the number of patients in the test arm who underwent the following non-invasive tests were: CTCA (674), FFRCT (220), stress echo (13), perfusion scan (4), stress MRI (15), exercise ECG (27). The number of patients in the reference arm who underwent these tests were: CTCA (460), FFRCT (9), stress echo (124), perfusion scan (34), stress MRI (20), and exercise ECG (99). A total of 22% fewer patients in the test group had invasive coronary angiography (ICA) compared to the reference group (136 vs. 175, p=0.01). There was no significant difference in the rates of MACCE or revascularization.
"Results from FORECAST indicate that CTCA and FFRCT as a frontline strategy may not be associated with the financial savings projected from observational data by NICE," said Nick Curzen, BM (Hons), PhD, Professor of Interventional Cardiology, University of Southampton, United Kingdom. "However, the reduction in invasive coronary angiography is important and will be very attractive to patients. More data is needed to determine the optimal use for FFRCT in clinical practice."
TARGET FFR & DEFINE-FLOW
TCT 2020|LBCS III
In a field that has grown accustomed to the idea of physiological assessment—particularly fractional flow reserve (FFR) to guide decisions to perform or defer revascularization—new data released today take a deeper dive into what these measurements actually mean.
The studies—TARGET FFR and DEFINE-FLOW—arrived one after the other today in a late-breaking session at TCT Connect 2020.
TARGET FFR found that physiologically-guided PCI did not lead to a greater proportion of patients having an optimal FFR ≥ 0.90 in treated vessels; that said, the strategy did reduce the proportion of patients with residual FFR ≤ 0.80. DEFINE-FLOW, meanwhile, brought coronary flow reserve (CFR) into the picture as a potential add-on to FFR before PCI; though complicated, the study essentially found that even high flow on CFR didn’t negate the importance of low FFR in determining which cases could safely be deferred.
“We need to pay more attention to the precise physiology that we’re measuring and what it means,” DEFINE-FLOW investigator K. Lance Gould, MD (UT Health Science Center, Houston, TX), urged when speaking to the press yesterday.
Indeed, physiological testing is not only a research tool. Its results carry clinical consequences. Presenting the TARGET FFR results in TCT’s “Main Arena,” Damien Collison, MB BCh (Golden Jubilee National Hospital, Glasgow, Scotland), said that subpar PCI results may explain why up to 38% of patients still report angina 1 year after treatment.
He pointed out that registry data have shown high variability in final FFR, with anywhere from 21% to 100% of patients achieving values of ≥ 0.90, which has been associated with lower risks of repeat PCI and MACE. “Perhaps more concerning,” said Collison, anywhere from less than 1% to 36% of patients have post-PCI FFRs of 0.80 or below, he noted.
Collison and colleagues enrolled 260 patients at their center between March 2018 and 2019, randomizing them after angiographically successful PCI to receive FFR-guided optimization or blinded FFR assessment.
In the guided group, FFR pullback of stented vessels suggested targets for further optimization in 60 of the 131 patients (46%), with operators deciding to perform additional postdilation and/or stenting in 40 of these individuals (66%). These steps increased mean post-PCI FFR from 0.76 to 0.82, with a larger increase seen with stenting than postdilation, while CFR rose from 3.0 to 4.0 (P = 0.02).
“Not unexpectedly, in an as-treated analysis, additional PCI optimization measures were associated with increased procedure duration [plus] higher doses of radiation, contrast, and adenosine,” Collison noted, adding that there was “no signal of excess harm” related to complications.
Overall, final FFR ≥ 0.90 trended higher, but just numerically so, with FFR-guided optimization (38.1% vs 28.1%; P = 0.099). FFR values ≤ 0.80, though, were significantly less common when post-PCI FFR informed treatment (18.6% vs 29.8%; P = 0.045). And in line with earlier registry data, mean FFR levels were lower when the target vessel was the LAD versus the left circumflex or right coronary arteries (0.80, 0.92, and 0.91, respectively; P < 0.001).
Session moderator Gregg W. Stone, MD (Icahn School of Medicine at Mount Sinai, New York, NY), followed up by asking why operators chose not to pursue further optimization for one-third of the patients whose FFR suggested room for improvement. In most instances, Collison replied, it came down to worries about the safety of high-pressure balloons after stenting, and in some cases imaging guidance may have suggested additional steps were unnecessary.
Allen Jeremias, MD (St. Francis Hospital, Roslyn, NY), lead author of the DEFINE-PCI trial, said he was “a little bit shocked” to see that so few patients had optimal FFR after angiographic guidance alone. “It really reinforces the importance of doing physiology before and after to guide PCI,” he commented.
Over the past few years, there’s been growing interest in better understanding microvascular abnormalities, Stone pointed out. “So it’s a real pleasure now to look at one of the first real scientific studies to examine how important these issues are,” he said, introducing DEFINE-FLOW.
The trial’s hypothesis was that, when
treated medically, lesions with abnormal FFR ≤ 0.80 but intact CFR ≥ 2.0
would have noninferior outcomes “compared to lesions where the FFR and
CFR were both intact,” Nils Johnson, MD (UT Health Science Center), said
in his presentation today.
Enrolling from 12 sites in six countries, DEFINE-FLOW researchers studied 430 patients who among them had 533 stable lesions that underwent simultaneous FFR and CFR assessment. Only lesions with both FFR ≤ 0.80 and CFR < 2.0 underwent PCI. All other variations, including when FFR indicated poor flow but CFR did not, meant that patients received medical therapy and PCI was deferred.
At 2 years, MACE (primary endpoint; all-cause death, MI, and revascularization) rates differed across the four possible combinations, with the lowest rate seen in patients both FFR and CFR negative and the highest in those both FFR and CFR positive. Target vessel failure (MI and revascularization) again was lowest in the patients who had the best FFR and CFR values; it was highest in patients who had low FFR but intact CFR.
Lesions in vessels that had abnormal FFR values of ≤ 0.80 and normal CFR values of ≥ 2.0 thus carried an absolute 5% higher 2-year risk of MACE than those showing normal values for both FFR and CFR. This difference in MACE risk did not reach significance for noninferiority (P = 0.065)—in other words, FFR mattered even in the face of normal CFR.
Johnson cautioned, though, there were limitations to consider—not only was the study observational, in particular, there was no comparison of treatment for lesions with positive FFR but negative CFR. This “really excludes our ability to make causal statements,” he said. Nor were operators and patients blinded to physiology results.
“DEFINE-FLOW is a hypothesis-generating study,” Johnson concluded, adding, “What this study has taught me, personally at least, is that we really need to distinguish between flow to the downstream myocardium and the intracoronary pressure that acts against the local plaque. I think it’s been natural to assume that the ability of the myocardium to increase its flow would really dominate symptoms and clinical events. What DEFINE-FLOW suggests is that local stress at the level of the stenosis contributes even more to the natural history of atherosclerosis.”
Gould said in the press conference that “this small study is quite important for several, perhaps unclear reasons.”
Its results undermine the idea that adequate CFR might overcome inadequate FFR, allowing for PCI deferral. Yet DEFINE-FLOW’s results are consistent with what has been seen in prior studies of quantitative perfusion on PET imaging, he explained. “The explanation mechanistically may be that the high stress flow, or CFR, through a mild-to-moderate stenosis or diffuse disease with no stenosis, causes a fall in coronary pressure. The fluid dynamic equations relating coronary flow pressure predict heterogeneous arterial wall stresses associated with plaque instability, subendocardial ischemia, or both.
“Consequently, the high-flow lesion with low FFR may indicate less-severe stenosis but still vulnerable plaque compared to low-flow lesions with low FFR, which may have still higher risk of plaque instability,” Gould continued.
Better understanding of which FFR/CFR thresholds are associated with mortality and morbidity could inform trial design, “potentially counterbalancing the ISCHEMIA and COURAGE trials that [relied on] precise quantitative severity of stenosis,” he said.
Roxana Mehran, MD (Icahn School of Medicine at Mount Sinai), moderating the press conference, said outright that she found the study “a little bit confusing.” Despite lack of noninferiority, in a statistical sense, Mehran drew attention to the cases with abnormal FFR ≤ 0.80 but intact CFR ≥ 2.0 as the main area for future research, asking: “Do we even have enough patients [in DEFINE-FLOW] to make very important conclusions yet, because of the permutations of the differential measurements?”
What’s been clearly shown from large randomized trials—such as DEFER and FAME 2—is
that treating lesions with low FFR helps, Chad Rammohan, MD (Sutter
Health, Mountainview, CA), commented. What’s being explored here is
whether CFR may help guide which patients can safely defer PCI despite
having low FFR. “The trial is a little
bit confusing for me as an interventional cardiologist,” he said, as is
the “double negative”—that is, “not noninferior”—in the conclusion.
The overall message is, for now, that FFR should guide decision-making, Rammohan and Mehran agreed, as did Gould. “This study says that you need to trust the FFR primarily at this level of [CFR],” Gould said. “I invented CFR. I’d love to see it work. But I’m a pretty cold-blooded scientist. This trial is very small [and] there are many limitations.” He said a larger RCT is needed, as well as a look at various CFR thresholds.
Mehran, for her part, called for
additional studies of CFR, particularly in diffuse disease and in women.
“There could also be some gender issues regarding the microvasculature
that could have an important implication on the coronary flow reserve,”
she observed.
SOLVE-TAVI
TCT 2020|LBCS III
One-year results from the SOLVE-TAVI trial, a head-to-head comparison of the self-expandable CoreValve
Evolut R (Medtronic) and the balloon-expandable Sapien 3 (Edwards
Lifesciences) in patients with symptomatic severe aortic stenosis at
high risk for surgery, show the two valves to be comparable with respect
to a broad composite endpoint that includes all-cause mortality,
stroke, moderate/severe paravalvular leak, and the need for a new
permanent pacemaker.
Drilling down, though, study investigators revealed a confusing, potentially worrisome finding, reporting that the rate of stroke, one of the study's secondary endpoints, was significantly higher with the Sapien 3 valve: 6.9% versus 1.0% with CoreValve Evolut R (P = 0.002).
Hans-Josef Feistritzer, MD, PhD (Heart
Center Leipzig-University Hospital, Germany), who presented the results
during the late-breaking clinical science session at TCT Connect 2020,
said the rate of stroke observed with Sapien 3 in SOLVE-TAVI is higher
than what has been previously seen in other trials and observational
studies. In SOURCE 3, for example, the rate of stroke with Sapien 3 at 30 days and 1 year was 1.4% and 3.1%, respectively. In PARTNER 3, a trial of low-risk patients that also used the Sapien 3 device, the stroke rate was 1.2% at 1 year.
To TCTMD, Feistritzer said the higher
risk of stroke might be related to more severe aortic root calcification
among patients treated with Sapien 3, but he did note that baseline
characteristics, such as STS-PROM risk score, patient age, and frailty
measures, among others, were similar in both treatment arms.
“Of course, we need more post hoc
analyses, which are already planned, to analyze possible confounders of
this finding,” he said. Feistritzer added the study operators
historically implanted more CoreValve devices and that experience with
Sapien 3 might be a potential factor explaining the higher stroke rate.
In a landmark analysis between 30 days and 1 year, there was no
statistical difference in the risk of stroke between the two devices, he
said.
Susheel Kodali, MD (NewYork-Presbyterian Hospital/Columbia University Irving Medical Center, New York), one of the moderators during a press conference where the results were presented, said head-to-head comparisons of TAVR devices are important for the field but that one of the challenges with such studies is the interaction with patient anatomy. “Each TAVR device interacts with the anatomy differently—how it crosses the arch and how it interacts with the annulus, the calcium, the root, everything—so there may be significant differences between the groups,” he said.
Another concern, said Kodali, is that SOLVE-TAVI is a relatively small study with 438 patients. “That sort of lends itself to random events, and although I understand the [statistical] significance, a stroke rate of 6.9% compared with registries or trials that had a thousand patients with neurological assessment where the stroke rate was much lower—there’s something that we’re missing [here]. I think the post hoc analysis will be important in looking at this.”
Following the late-breaking presentation, panelist Megan
Coylewright, MD (Dartmouth-Hitchcock Medical Center, Lebanon, NH), like
all the others, zeroed in on the higher rate of stroke. In the
intermediate-risk trials, stroke rates range from 3% to 5%, with little
difference between the two platforms (although those studies weren’t
comparing CoreValve with Sapien directly), she said.
Gregg Stone, MD (Icahn School of Medicine at Mount Sinai, New York), also a panel discussant, stressed the study size. “Just from a statistical point of view, there was a sevenfold difference in stroke in a 400-patient randomized trial, which is vastly underpowered to detect small differences in stroke, let alone large differences,” he said, noting that other studies have suggested higher risks of stroke with CoreValve; none of those studies were definitive either. “I think I can say pretty confidently there is not a sevenfold difference in stroke rates between the devices. I’m willing to take that one to the bank.”
SOLVI-TAVI was a 2 x 2 factorial trial that randomized patients with symptomatic severe aortic stenosis to TAVR with CoreValve Evolut R versus Sapien 3 as well as to a strategy of local versus general anesthesia. Patients in the trial were approximately 82 years old, and the mean STS risk score was 7.7% with Evolut R and 7.6% with Sapien 3.
With respect to valve strategy, there was no difference in the primary composite endpoint at 1 year, nor any significant difference in the risk of all-cause mortality (17.6% with Evolut R vs 17.0% with Sapien 3; P = 0.88). Rates of moderate/severe paravalvular leak were similar in both arms (7.0% with Evolut R vs 4.5% with Sapien 3; P = 0.35). Although the rates of permanent pacemaker implantation were similar between the devices, they were relatively high in both study arms: 24.7% with Evolut R and 20.2% with Sapien 3 (P = 0.25).
“Even with the balloon-expandable
[valve] at 1 year, a new pacemaker rate of 20% seems a little bit high,”
said Kodali. He noted that the SOLVE-TAVI population is relatively
high-risk based on the STS score, akin to patients randomized in the
pivotal high-risk trials with CoreValve and Sapien. Nonetheless, Kodali
said he doesn’t have a good answer to explain the high pacemaker rates.
Feistritzer conceded that pacemaker rates in SOLVE-TAVI were elevated, both at 30 days and 1 year. In the FORWARD registry of patients with Evolut R, the pacemaker rate was 19.7%, which isn’t out of line with what was observed with the self-expanding valve in SOLVE-TAVI. As for the high pacemaker rate with Sapien 3, however, Feistritzer said the reason might be attributed to older implantation techniques. “This study started many years ago, and maybe at this time point, implantation [depth] was lower than it is today,” said Feistritzer.
Stone, along with Samir Kapadia, MD (Cleveland Clinic, OH),
agreed that the higher pacemaker rates, particularly with Sapien 3, and
the higher rate of stroke with the device, would go hand-in-hand if
operators had more experience with CoreValve, as suggested by
Feistritzer.
In the sedation arm of the trial, there
was no difference in all-cause or cardiovascular mortality at 1 year in
patients who had local or general anesthesia at the time of their
procedures. Rates of stroke, MI, infection need for antibiotics, acute
kidney injury, and other safety measures were all equivalent between the
two sedation strategies.
In addition to safety, Feistritzer said conscious sedation translates into shorter hospital and ICU stays, and while they didn’t perform a cost analysis, one is planned, and he suspects it will favor local anesthesia. Overall, the study shows that conscious sedation can be performed in the “majority” of patients undergoing TAVR, he added.
Chad Rammohan, MD (Sutter Health, Mountainview, CA), who wasn’t involved in the study, commended the European researchers for performing a randomized trial of local versus general anesthesia, saying the use of general anesthesia goes back to the earliest days of TAVR. Registry data suggests that as many as 70% of transfemoral TAVR procedures are now performed with local anesthesia and data suggests trends toward improved clinical outcomes with no price to pay for leaving general anesthesia behind.
“I’ve converted completely to conscious sedation,” said Rammohan.
Kodali said that, while they also do the majority of their cases with conscious sedation, he still thinks local anesthesia can play a role.
“It’s understanding what anatomies
might benefit,” he said. “What is the risk of anesthesia? What is the
risk of TAVR? Those are separate, right? If you had a low EF/elderly
patient with [chronic obstructive pulmonary disease], the risk of
anesthesia is higher. But if I have a younger guy with a bicuspid valve
with heavy calcium, the risk of anesthesia is low, but my risk of
getting a poor result with TAVR is higher. In that patient where I want
to optimize the result, I’ll use [transesophageal echocardiography]
because the anesthesia risk is low. I think you have to take all those
factors into account.”
The goal is an optimal TAVR result at
the lowest risk possible, and each operator will have their own
preference for local or general anesthesia based on that equation, said
Kodali.
STS/ACC TVT Registry
TCT 2020|LBCS III
Information from two large US databases suggest that use of the Sentinel cerebral protection system (Boston Scientific) during TAVR reduces the risk of stroke, although experts continued to call for randomized trials to definitively settle the issue.
In the Society of Thoracic Surgeons/American College of Cardiology (STS/ACC) TVT Registry, the rate of in-hospital stroke was not significantly lower when the Sentinel device was used according to an instrumental-variable analysis (1.39% vs 1.54%; RR 0.90; 95% CI 0.68-1.13), David Cohen, MD (Kansas City, MO), reported during a late-breaking clinical science session at the virtual TCT Connect 2020.
A secondary propensity-weighted analysis of the TVT data, however, indicated that cerebral protection was associated with a reduction in in-hospital stroke (1.30% vs 1.58%; RR 0.82; 95% CI 0.69-0.97).
Consistent with the propensity-weighted analysis, a study involving data from the National Inpatient Sample presented by Michael Megaly, MD (Banner University Medical Center, Phoenix, AZ), during the TCT “Best Abstracts” session yesterday showed that after propensity-matching, Sentinel use was associated with a lower risk of in-hospital ischemic stroke (1.0% vs 3.8%; OR 0.24; 95% CI 0.09-0.62).
Speaking to the TVT Registry data, Cohen said at a press briefing that “both the secondary propensity-adjusted analysis and the confidence interval for the primary analysis are consistent with a possible modest reduction in stroke, with a relative risk reduction of about 20% and a number needed to treat, at least in this study, of about 300 for preventing what I consider to be a major stroke.”
He and others indicated that there’s a need for more-definitive randomized data to determine the impact of cerebral embolic protection devices like Sentinel on patient outcomes. Highlighting that need was the REFLECT II trial presented earlier at the meeting; in that study, another protection device, TriGUARD 3 (Keystone Heart), failed to demonstrate a benefit in terms 30-day stroke or death.
A trial designed to assess any potential clinical effects of cerebral embolic protection—PROTECTED TAVR, which will enroll about 3,000 patients and is estimated to be completed in July 2022—is currently randomizing patients to TAVR with or without the Sentinel device.
The TVT Registry analyses “support clinical equipoise and they provide a strong rationale for the ongoing large-scale randomized trials to test whether embolic protection devices truly provide meaningful clinical benefit—that is, reduced stroke or improved neurocognitive function for patients undergoing TAVR,” Cohen said.
Despite improvements in TAVR technology and technique, increased operator experience, and refined patient selection, stroke remains a problem, occurring in 2% to 5% of cases. Cerebral protection devices like Sentinel, cleared by the US Food and Drug Administration in June 2017, were developed to lessen that risk. There are, however, no randomized studies definitively showing that the devices—which capture debris bound for the brain in nearly all patients—improve clinical outcomes.
Cohen et al explored the potential clinical impact using data on transfemoral TAVR procedures performed in 2018 and 2019 included in the STS/ACC TVT Registry. They excluded emergent procedures as well as those involving alternative access, done at sites performing fewer than 20 TAVRs each year, and involving concomitant mitral interventions.
Over the 2-year study period, the proportion of hospitals offering embolic protection increased from 7% to 28%, and the proportion of patients receiving protection increased from 5% to 13%. There was substantial variability across sites, however, with two-thirds of hospitals using no embolic protection and about 5% using protection devices in more than half of procedures.
In the instrumental-variable analysis, Sentinel use was not associated with risk of any of the outcomes examined, including stroke, death, and major bleeding in the hospital and stroke and death at 30 days.
In the secondary propensity-weighting analysis, however, use of cerebral embolic protection was associated with lower rates of in-hospital stroke and death or stroke (2.1% vs 2.5%; RR 0.84; 95% CI 0.73-0.98), as well as 30-day stroke (1.9% vs 2.2%; RR 0.85; 95% CI 0.73-0.99) and death (1.7% vs 2.2%; RR 0.78; 95% CI 0.64-0.95).
Though the findings appear to conflict, “in some ways, these two analyses are more similar than different,” Cohen said, noting that the confidence intervals are wider in the instrumental-variable analysis. “These results are actually not that inconsistent with one another.”
Megaly et al explored the impact of the Sentinel device using data from the National Inpatient Sample. Their analysis included 36,220 patients who underwent TAVR in the last three quarters of 2017; Sentinel was used in 525 of them (1.4%). Cerebral protection was more frequently used at large teaching hospitals. Patients in whom Sentinel was used were less likely to have PAD and a history of coronary revascularization, and none had carotid disease, which is a contraindication to use.
Sentinel patients were propensity-matched to 1,050 patients who underwent unprotected TAVR. Those who were protected had a lower risk of in-hospital ischemic stroke, as well as a lower risk of in-hospital death (0 vs 1%; P = 0.036). The mortality finding should be interpreted with caution because of the study’s retrospective nature, Megaly said.
There were no differences between groups for vascular complications, blood transfusions, renal complications, length of stay, and the likelihood of discharge to a nursing facility, although index hospitalization costs were higher in the Sentinel group ($47,783 vs $44,578; P = 0.002).
On multivariate analysis, several factors independently associated with a higher risk of post-TAVR ischemic stroke emerged: history of carotid disease, PAD, atrial fibrillation or flutter, older age, bicuspid aortic valve, and female sex. All of these factors, Megaly said, would suggest a need to use cerebral embolic protection.
In a panel discussion during the press briefing, Susheel Kodali, MD (NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY), who co-led the SENTINEL trial, questioned Cohen about how his analyses could account for all confounders. For example, he said, “the sites that use Sentinel may be more concerned about stroke and maybe they’re more focused on the neurologic analysis and do neurologic checks.”
Cohen said the data suggested that might be an issue, so they adjusted for the rate of stroke at the sites in 2017, before anybody started using embolic protection. He noted that it’s possible the propensity-weighted analysis was less affected by that potential confounding.
What Cohen said he would take away from his study is that equipoise exists to support randomization in ongoing trials. In addition, “what is sobering to me is that even if there is benefit here, the benefit may be less than we think,” he said, adding that the 20% to 25% relative risk reduction suggested by the analysis is “a little bit sobering for a device that’s supposed to protect three out of the four great vessels.”
Commenting for TCTMD, Dharam Kumbhani, MD (UT Southwestern Medical Center, Dallas, TX), said Cohen’s study is “helpful to kind of see the overall landscape for the field. And I agree with his conclusion that it’s probably appropriate to have an adequately powered trial that assesses clinical endpoints with this device.”
Asked whether the totality of the evidence on cerebral protection devices justifies use in 13% of patients undergoing TAVR, Kumbhani responded, “The use is a little bit higher than what I think the evidence suggests. The evidence does not support widespread use of this device.”
He said that at his center, Sentinel is used primarily in patients with prior stroke and in those with heavy calcification in the ascending aorta and aortic arch.
Because of the inherent
limitations of observational analyses, the TVT Registry data are not
likely to push operators one way or another in terms of how they’re
currently using the Sentinel device, Kumbhani said. “It is FDA-approved
and I think operators will probably want to use it in the right patient
population.”
At the press briefing, Chad Rammohan, MD (Sutter Health, Mountain View, CA), said about the impact of the Sentinel observed in the TVT Registry, “The fact that the absolute risk reduction is so low and the number needed to treat is so high, it allows us to realize that they’re pretty much equal based on this data and we have to wait for the big trial. And if you’re not using it, it’s probably okay to not use it, and if you’re using it, it’s probably okay to use it until the big trial comes out.”
IREMMI
TCT 2020|LBCS IV
For patients with acute mitral regurgitation (MR) following myocardial infarction, including those who present with and without cardiogenic shock, treatment with MitraClip (Abbott) may be a viable option for this high-risk cohort who have no surgical alternative, according to a small, retrospective series.
Overall, investigators reported that percutaneous repair was associated with “acceptable rates of death and rehospitalization,” including in those with acute MR who presented in cardiogenic shock, as well as improvements in MR grade and NYHA functional class.
After a median follow-up of 7 months, 16.3% of patients with acute MR and cardiogenic shock following MI died after treatment with MitraClip compared with 9.3% of patients who didn’t develop cardiogenic shock (P = 0.377). The combined rate of death or rehospitalization due to heart failure was 28% and 25.6% in the patients with acute MR with and without cardiogenic shock after MI (P =0.793).
Presenting the IREMMI results during today’s late-breaking clinical science session at TCT Connect 2020, Rodrigo Estévez-Loureiro, MD, PhD (University Hospital Alvaro Cunqueiro, Vigo, Spain), explained that MR stemming from papillary muscle rupture was infrequent in their series, with most of it due to acute ventricular remodeling and restriction of the posterior mitral leaflet.
Mortality for patients with severe MR after MI is extremely high, he said.
“Until recently, surgery was the only alternative, but there have been some reports of MitraClip being used in this scenario,” said Estévez-Loureiro. However, all patients in the present study had been turned down by the surgical team, leaving medical therapy the only option. “We know medical therapy is associated with the highest rate of mortality—50% at 30 days,” for these patients, he said.
In this registry, investigators
included consecutive patients with acute MR complicating MI treated with
MitraClip at 18 centers in Europe, North America, and Israel between
2016 and 2020. Over 4 years, the group enrolled just 93 patients,
including 50 who presented in cardiogenic shock and 43 without shock.
For those in cardiogenic shock, MitraClip to treat acute MR was
implanted 24 days after presenting to hospital for MI. For those not in
cardiogenic shock at presentation, MitraClip was implanted 33 days after
the MI.
At baseline, 86% and 79% of shock and nonshock patients had MR grade 4. Immediately after MitraClip implantation, the majority of patients had a significant improvement in MR grade, with more than 60% getting to an MR grade of 0-1. These benefits were observed in shock and nonshock patients. At 3 months, the results were similar, with again no difference in MR improvement seen between shock and nonshock patients treated with MitraClip. NYHA functional class was also improved: while the majority were in NYHA class IV at baseline, 80% and 85% of the cardiogenic shock and noncardiogenic shock patients, respectively, were in NYHA class I or II at follow-up (P = 0.608 for difference between groups).
With respect to clinical events,
Estévez-Loureiro said cardiogenic shock was not associated with an
increased risk of mortality or hospitalization for heart failure.
“The only variable that was
significantly associated with this composite endpoint was procedural
success,” he said. As such, cardiogenic shock, if its adequately
supported, doesn’t influence short- and mid-term outcomes of
percutaneous repair with MitraClip and shouldn’t preclude its use, said
Estévez-Loureiro.
Dee Dee Wang, MD (Henry Ford Health System, Detroit, MI), who spoke during a media briefing where the results were announced, praised the investigators for the study, noting that it’s challenging to collect data on patients at such high risk. She questioned why there was a long delay between MI presentation and treatment of acute MR. In response, Estévez-Loureiro said the strategy of most centers had been to cool down the cardiogenic shock patient and then provide mechanical support and medical therapy before considering percutaneous repair with MitraClip.
“In the end, after two or three failed
therapies, then it becomes an option,” he said. For the cardiogenic
shock patient, he also stressed stabilization is critical.
Cardiac surgeon Michael Borger, MD, PhD
(Leipzig Heart Center, Germany), who moderated the late-breaking
science session, said that when compared to ACS patients with acute MR
sent for surgery, the mortality rate in the IREMMI series “is very, very
respectable.” Estévez-Loureiro said researchers are currently
collecting and analyzing outcomes of patients with severe MR following
MI treated with medical therapy and surgery to serve as control arms for
comparisons with MitraClip.
“I don’t want to unveil our results,
but they’re quite interesting, and hopefully we’ll be releasing them in a
few months,” said Estévez-Loureiro.
Panelist Jeroen Bax, MD (Leiden
University Medical Center, the Netherlands), attributed the solid IREMMI
outcomes to the expertise of the hospitals and physicians participating
in the registry. He questioned whether the geometry of the ventricle
had an impact on the efficacy of the MitraClip to treat the mitral
valve. LV dimensions, relative to MR severity, have dominated
discussions about the MitraClip’s efficacy in clinical trials to date.
Estévez-Loureiro said that geometry was not a factor.
“All these procedures were done in highly experienced centers, so people were quite used to treating complex anatomies,” he said. With the mechanism underlying MR being LV remodeling and restriction of the posterior leaflet, this might make it a little bit easier for MitraClip implantation. Nonetheless, even in the few patients with papillary muscle rupture, operators were also successful implanting the device, Bax noted.
SCOPE I
TCT 2020|LBCS IV
There were no significant differences in clinical and functional outcomes in patients with severe aortic stenosis undergoing transfemoral TAVR with the self-expanding ACURATE neo compared with the balloon-expanding SAPIEN 3, said researchers presenting the SCOPE I trial Oct. 17 during TCT 2020. The trial did not meet criteria for non-inferiority.
Thomas Walther, MD, et al., looked at 739 patients (mean age 82.3 years) who were randomized to either the ACURATE neo (N=372) or SAPIEN 3 (N=367). One-year follow-up was completed for 96% of patients in the ACURATE neo group and 97% in the SAPIEN 3 group.
The primary outcome was all-cause death or disabling stroke at one year, which occurred in 12% of the ACURATE neo group and 9.4% in the SAPIEN 3 group. Researchers also observed "the rate of paravalvular regurgitation remained higher, but the hemodynamic profile [was] better with lower transprosthetic mean gradients and higher effective orifice area at one year in patients treated with ACURATE neo."
Based on the findings, SCOPE 1 investigators said that extended follow-up data "will be crucial to determine the impact of the differential valve performance on long-term outcomes."
TRANSIT
TCT 2020|LBCS IV
"The expanding adoption of TAVR to relatively young and/or low-risk patients will conceivably result in an increasing number of patients with a degenerated bioprosthetic valve," said Luca Testa, MD, PhD, presenting results of the TRANSIT study Oct. 17 during TCT 2020. "These patients may be safely and successfully treated by means of a second transcatheter procedure."
The TRANSIT study is based on registry data from more than 172 patients at 28 centers from Europe, North America, South America and the Middle East who underwent a second TAVR due to a degenerated transcatheter aortic bioprosthesis. Patients were analyzed based on the mode of failure of the first bioprosthetic valve (57 stenosis, 97 regurgitation, 18 mixed).
Results showed an overall mortality rate at one year of 10% and a cardiovascular mortality rate of 5.8%. Low rates of major clinical events were also observed, as were significant clinical benefits based on the rate of hospitalizations and NYHA class.
"The impact of this study for patients affected by degenerated aortic bioprosthesis is considerable as they have very limited options and an unfavorable prognosis," said Testa, et al. "In terms of delivery of care, this study provides evidence on a treatment that will be considered for a continuously growing number of patients."
PARTENER 2
TCT 2020|LBCS IV
Mortality at five years following valve-in-valve TAVR is comparable with that seen in native TAVR intermediate-risk patients, based on five-year follow-up from the PARTNER 2 trial looking at clinical outcomes, valve function and durability function presented Oct. 17 during TCT 2020.
John G. Webb, MD, FACC, et al., analyzed data from 365 patients in whom valve implant was completed. They noted that in survivors, early improvement in functional status and quality of life were maintained for five years. Additionally, rates of heart valve disease and bioprosthetic valve fracture were consistent with those reported for native SAPIEN XT valves in patient with intermediate risk.
Study investigators noted that these new findings add to the growing body of evidence regarding valve-in-valve TAVR, which has been associated with acceptable mortality, improved valve hemodynamics, and excellent quality of life outcomes at three years. However, limited data have been available to date on longer-term clinical outcomes, valve function and durability.
Refer to
Effect of Pre-Hospital Crushed Prasugrel Tablets in Patients with STE...
Circulation. | Oct 15,2020
Percutaneous Coronary Intervention for Vulnerable Coronary Atherosclerotic ...
JACC | Oct 15,2020
Closure of Iatrogenic Atrial Septal Defect Following Transcatheter Mitra...
Circulation. | Oct 16,2020
Three-Year Outcomes of the ULTIMATE Trial Comparing Intravascular ...
JACC: Cardiovascular Interventions | Oct 16,2020
Blinded Physiological Assessment of Residual Ischemia After Successful A...
JACC: Cardiovascular Interventions | Oct 16,2020