


-
-
Scientific LibraryAcute Coronary Syndrom ASCVD Prevention Bifurcation Stenting Cardio-Oncology Congestive Heart Failure DAPT Duration Drug Coated Balloon Fractional Flow ReserveNewsCases VideosE-LearningIndustry Insights
- LIVE
-
 Article Link
Article Link

The ASCEND Trial: Who Benefits With Aspirin?
Richard Haynes; Marion Mafham, MB ChB; Jane Margaret Armitage, MBBS; Louise Bowman, MBBS,MD
Although aspirin has been used since the 19th century, it was not until the late 20th century that randomized evidence assessing its use in the secondary prevention of cardiovascular disease became available. A meta-analysis of these trials demonstrated that aspirin reduces the risk of non-fatal occlusive vascular events (among people with a history of such disease) by 19% (95% confidence interval [CI], 13-25%).1 This proportional reduction translated into an annual absolute reduction of 1.5% (i.e., 15 fewer events for every 1,000 patients treated for 1 year). Aspirin also causes bleeding (an inevitable consequence of its antiplatelet effect and also its potential to injure the upper gastrointestinal tract), but it is widely accepted that the benefits outweigh the risks among people with prior occlusive vascular disease unless they are at particularly high risk of bleeding.
Aspirin has also been tested among people with no prior history of vascular disease, where the risks and benefits are much more similar in magnitude (on the absolute scale). Overall, in the primary prevention studies that largely included people in middle or older age with or without cardiovascular risk factors, the annual absolute reduction of serious vascular events is 0.07%, and the annual absolute increase in major bleeding is 0.04% (0.01% and 0.03% for intra- and extra-cranial bleeding respectively).2 Aspirin is therefore not routinely recommended for such patients.
Primary and secondary prevention patients differ substantially in their risk of further vascular events, so there has been significant interest in populations at moderate risk (i.e., in between these two extremes). Such populations include the elderly, patients with chronic kidney disease, and patients with diabetes mellitus. The latter group was the focus of ASCEND (A Study of Cardiovascular Events in Diabetes).3,4
ASCEND recruited 15,480 participants with diabetes mellitus but no prior history of occlusive vascular disease who were randomized between aspirin 100 mg once daily and matching placebo (and, separately, between a 1-gram capsule containing omega-3 fish oils daily and placebo in a 2 x 2 factorial design).4 The primary outcome was serious vascular events, defined as the composite of non-fatal myocardial infarction, non-fatal non-haemorrhagic stroke, transient ischaemic attack (TIA), and vascular death excluding confirmed intracranial haemorrhage. ASCEND was unique in its design and methodology: participants were identified from registers of patients with diabetes (held by hospitals, other regional National Health Service bodies, or primary care physicians) and recruited and followed-up entirely by mail.5 Study treatment was sent to participants by mail (in specially designed packs), and follow-up information was collected by regular mailed (and, later in the trial, web-based) questionnaires, supplemented by information from national death, cancer, and hospitalisation registries. These streamlined and highly cost-effective procedures yielded mean adherence of 70% (very similar to previous aspirin trials) and complete follow-up in 99.1% of participants.3
The ASCEND participants are typical of a Western diabetes population: mean age was 63 years, and 63% were male. Mean body mass index was over 30 kg/m2, and median duration of diabetes was 7 years. After 7.4 years mean follow-up, 658 (8.5%) of those allocated aspirin versus 743 (9.6%) of those allocated placebo had at least 1 serious vascular event, producing a rate ratio of 0.88 (95% CI, 0.77-0.97). The effect on major bleeding (a composite of intracranial bleeding, sight-threatening eye bleeding, serious gastrointestinal bleeding, and other bleeding requiring hospitalisation) was opposite: 313 (4.1%) versus 245 (3.2%); rate ratio 1.29 (95% CI, 1.09-1.52). The absolute effects were therefore similar: a 1.1% reduction in serious vascular events offset by a 0.9% increase in major bleeding. The proportional effects (for both vascular and bleeding outcomes) were similar in the different types of participants studied.
Because the absolute effects depend on both the proportional treatment effect and the baseline risk of the outcome in question, a key question is whether there is a group of participants at sufficient baseline risk of vascular events that the benefits outweigh the risks (as is assumed to be the case in secondary prevention). Using the placebo arm, a vascular risk score was constructed within the ASCEND population so that participants could be grouped according to their cardiovascular risk and the absolute effects of aspirin examined. However, as had been described in the earlier meta-analysis, bleeding risk was strongly correlated with vascular risk (with many shared risk factors) (Table 1) so in all subgroups of vascular risk (<5%, 5% to <10%, ≥10% risk of serious vascular events, including TIA, over 5 years), the absolute benefits were approximately similar to the bleeding hazard (Figure 1). It could be argued that cardiovascular and bleeding events are not equivalent, but mortality associated with bleeding is substantial, and haemorrhagic strokes are on average more disabling (and more likely to be fatal) than ischaemic strokes.
Table 1: Rate Ratios (95% CI) Associated With Risk Factors for Major Coronary Events and Major Extracranial Bleeds in People With no Known Vascular Disease in Primary Prevention Trials.2

Figure 1: The Observed Absolute Effects of Aspirin Assignment on Serious Vascular Event or Revascularization and Major Bleed in Categories of Patients Sub-Divided by Vascular Risk
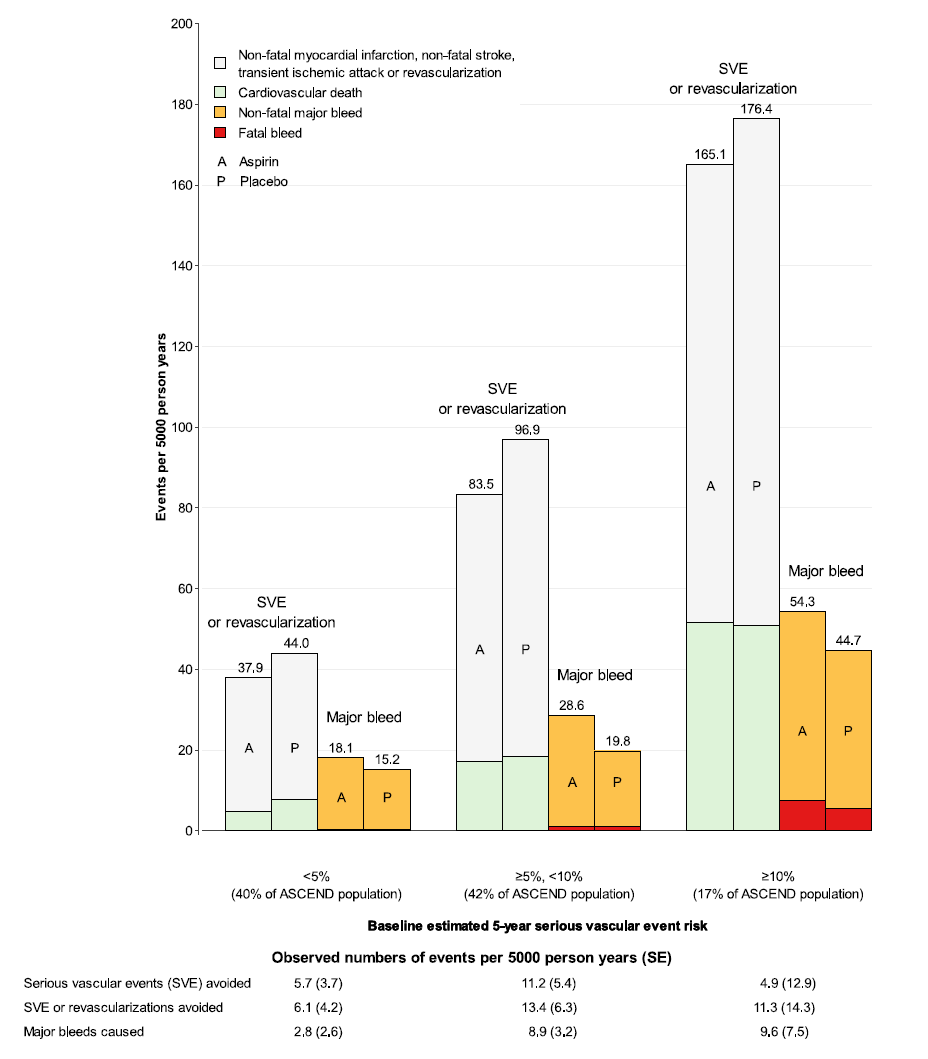
Figure 2: Vascular Event Rates in Different Populations and at Different Times

References
1. Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71-86.
2. Antithrombotic Trialists' Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849-60.
3. ASCEND Study Collaborative Group, Bowman L, Mafham M, et al. Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus. N Engl J Med 2018;379:1529-39.
4. Bowman L, Mafham M, Stevens W, et al. ASCEND: A Study of Cardiovascular Events iN Diabetes: Characteristics of a randomized trial of aspirin and of omega-3 fatty acid supplementation in 15,480 people with diabetes. Am Heart J 2018;198:135-44.
5. Aung T, Haynes R, Barton J, et al. Cost-effective recruitment methods for a large randomised trial in people with diabetes: A Study of Cardiovascular Events iN Diabetes (ASCEND). Trials 2016;17:286.
6. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet 2018;392:1036-46.
7. McNeil JJ, Wolfe R, Woods RL, et al. Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. N Engl J Med 2018;379:1509-18.
8. ISRCTN40920200: ATTACK: Aspirin to prevent a first heart attack or stroke in people with chronic kidney disease (ISRCTN Registry web site). 2019. Available at: https://doi.org/10.1186/ISRCTN40920200. Accessed March 22, 2019.
9. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. Circulation 2019;Mar 17:[Epub ahead of print].

http://www.cbsmd.cn Contact us by cbs@cbsmd.cn
Copyright ⓒ CBSMD Nanjing China. All rights reserved.



